Element Bromine - Br
Comprehensive data on the chemical element Bromine is provided on this page; including scores of properties, element names in many languages, most known nuclides of Bromine. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Bromine Menu
- Bromine Page One
- Bromine Page Two
- Bromine Page Three
Overview of Bromine
- Atomic Number: 35
- Group: 17
- Period: 4
- Series: Halogens
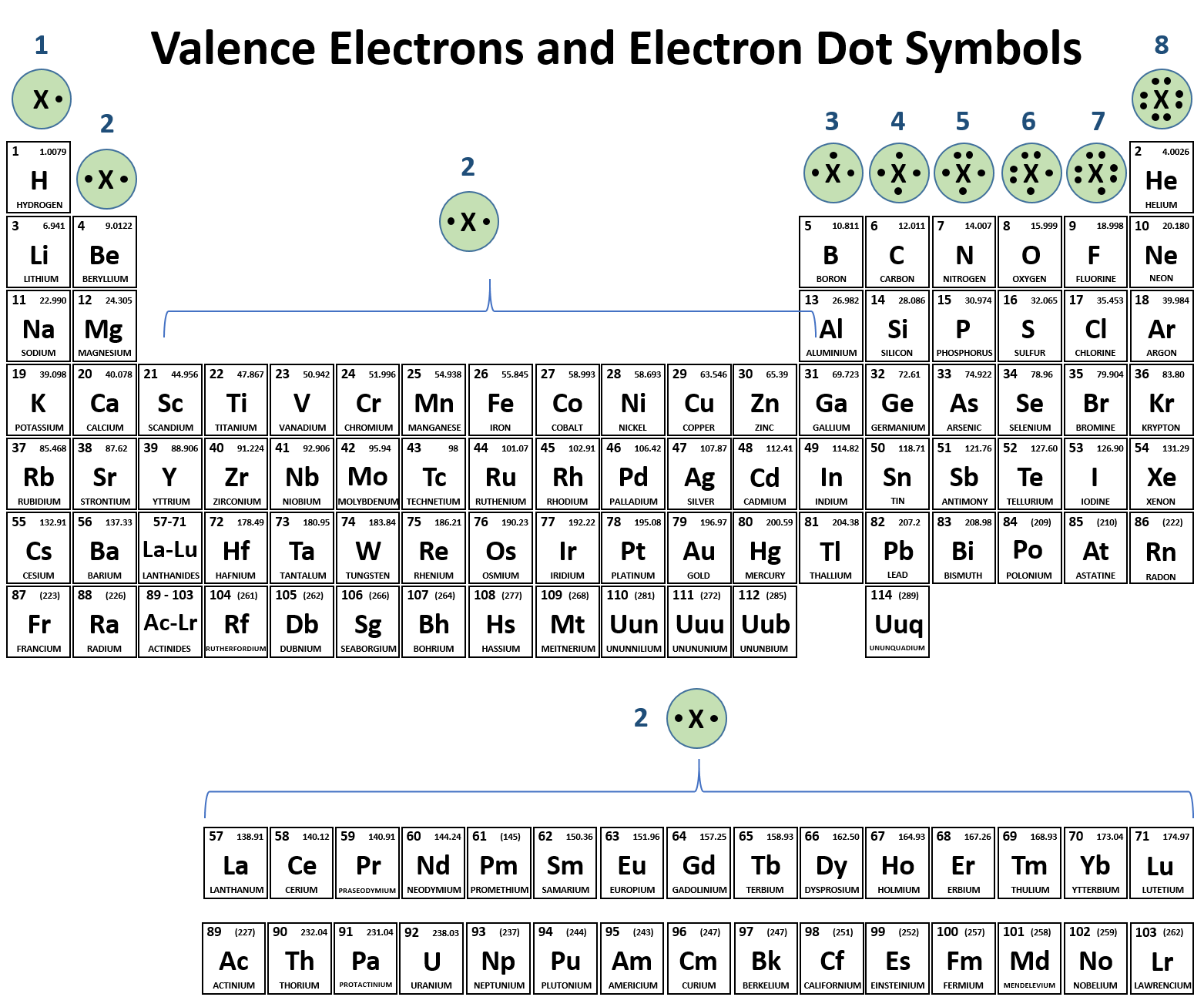
We already know that atomic number is defined as the number of protons and electrons in an atom. Of protons in Br = 35 No. Of electrons in Br = 35 ◆ Mass number of Bromine = 80. There are a total of 28 valence electrons for the BrF 3 Lewis structure. After determining how many valence electrons there are in BrF 3, place them around the central atom to complete the octets. Bromine is the least electronegative atom in the BrF 3 Lewis structure and therefore goes at.
- Bromine atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Since bromine has 7 valence electrons, the 4s orbital will be (a)This diagram represents the correct filling of electrons for the nitrogen atom.The orbital diagram for Bromine is Share to: Orbital notation.
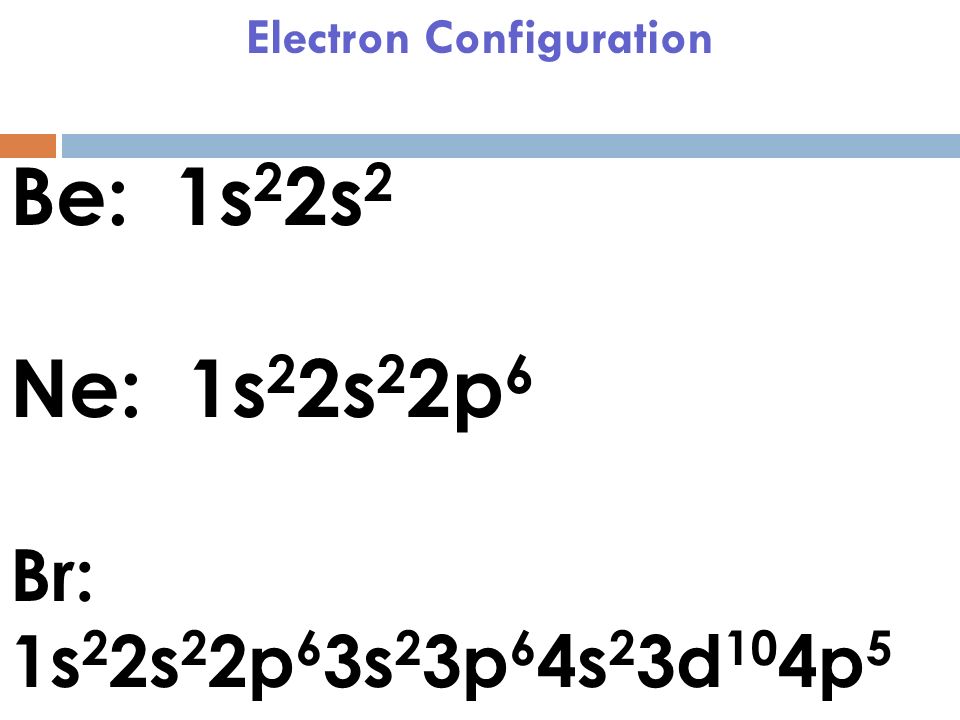
- Bromine has the electron configuration Ar3d 10 4s 2 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising agent, reacting with many elements in order to complete its outer shell.
Bromine's Name in Other Languages
- Latin: Bromum
- Czech: Brom
- Croatian: Brom
- French: Brome
- German: Brom - r
- Italian: Bromo
- Norwegian: Brom
- Portuguese: Bromo
- Russian: Бром
- Spanish: Bromo
- Swedish: Brom
Atomic Structure of Bromine
- Atomic Radius: 1.12Å
- Atomic Volume: 23.5cm3/mol
- Covalent Radius: 1.14Å
- Cross Section (Thermal Neutron Capture)σa/barns: 6.8
- Crystal Structure: Orthorhombic
- Electron Configuration:
- 1s2 2s2p6 3s2p6d10 4s2p5
- Electrons per Energy Level: 2,8,18,7
- Shell Model
- Shell Model
- Ionic Radius: 1.96Å
- Filling Orbital: 4p5
- Number of Electrons (with no charge): 35
- Number of Neutrons (most common/stable nuclide): 45
- Number of Protons: 35
- Oxidation States:±1,5
- Valence Electrons: 4s2p5
- Electron Dot Model
- Electron Dot Model
Chemical Properties of Bromine
- Electrochemical Equivalent: 2.9812g/amp-hr
- Electron Work Function:
- Electronegativity: 2.96 (Pauling); 2.74 (Allrod Rochow)
- Heat of Fusion: 5.286kJ/mol
- Incompatibilities:
- combustible organics (sawdust, wood, cotton, straw, etc.), oxidizable material, aqueous ammonia, hydrogen, acetylene, phosphorus, aluminum, titanium, mercury, potassium, other metals.
- Ionization Potential
- First: 11.814
- Second: 21.8
- Third: 36
- Valence Electron Potential (-eV): -7.35
Physical Properties of Bromine
- Atomic Mass Average: 79.904
- Boiling Point: 332.4K 59.25°C 138.65°F
- Coefficient of lineal thermal expansion/K-1: N/A
- Conductivity
- Electrical:
Thermal: 0.00122 W/cmK
- Electrical:
- Density: 3.119g/cc @ 300K
- Description:
- Heavy, red-brown, fuming liquid with a choking, irritating odor; causes tears
- Elastic Modulus:
- Bulk: 1.9/GPa
- Enthalpy of Atomization: 111.7 kJ/mole @ 25°C
- Enthalpy of Fusion: 5.29 kJ/mole
- Enthalpy of Vaporization: 15.46 kJ/mole
- Flammablity Class: Noncombustible Liquid
- Freezing Point:see melting point
- Heat of Vaporization: 15.438kJ/mol
- Melting Point: 266.05K -7.1°C 19.2°F
- Molar Volume: 25.62 cm3/mole
- Optical Refractive Index: 1.001132
- Physical State (at 20°C & 1atm): Liquid
- Realitive Gas Density (Air=1) = 5.51
- Specific Heat: 0.473J/gK
- Vapor Pressure = 5800Pa@-7.1°C
Regulatory / Health
- CAS Number
- 7726-95-6
- UN/NA ID and ERG Guide Number
- 1744 / 154
- RTECS: EF9100000
- NFPA 704
- Health: 4
- Fire:
- Reactivity:
- Special Hazard:
- OSHAPermissible Exposure Limit (PEL)
- 1 ppm = 6.54mg/m3 @ 25°C & 1 atm
- TWA: 0.1 ppm
- OSHA PEL Vacated 1989
- TWA: 0.1 ppm
- STEL: 0.3 ppm
- NIOSHRecommended Exposure Limit (REL)
- TWA: 0.1 ppm
- STEL: 0.3 ppm
- IDLH: 3 ppm
- Routes of Exposure: Inhalation; Ingestion; Skin and/or eye contact
- Target Organs: Respiratory system, eyes, central nervous system, skin
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 4.7
- Bone/p.p.m: 6.7
- Liver/p.p.m: 0.2-7
- Muscle/p.p.m: 7.7
- Daily Dietary Intake: 0.8-24 mg
- Total Mass In Avg. 70kg human: 260 mg
Who / Where / When / How
- Discoverer: Antoine J. Balard/ C. Löwig
- Discovery Location: Montpellier France/Heidelberg Germany
- Discovery Year: 1826
- Name Origin:
- Greek: brômos (stench).
- Abundance of Bromine:
- Earth's Crust/p.p.m.: 0.37
- Seawater/p.p.m.: 65
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): N/A
- Sources of Bromine:
- Occurs in compounds in sea water, Dead Sea, natural brines and salt-lake evaporates. World wide production estimated to be around 330,000 tons per year. Main mining areas are USA, Israel, UK, Russia, France and Japan.
- Uses of Bromine:
- Used for water purification (swimming pools), manufacture of ethylene dibromide (anti-knocking gasoline), bleaching, organic synthesis, solvent, analytical reagent, fire retardant for plastics, pharmaceuticals, shrink-proofing wool.
- Additional Notes:

Bromine Menu
- Bromine Page One
- Bromine Page Two
- Bromine Page Three
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Bromine - Br. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/25/2021
https://EnvironmentalChemistry.com/yogi/periodic/Br.html
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Br.html'>echo Periodic Table of Elements: Bromine - Br (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Bromine - Br is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
PLEASE, if you like an article we published simply link to it on our website do not republish it.
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner ProgramWhat Is The Number Of Electrons In Bromine
and our community of experts to gain a global audience for your work!Bromine (Br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or Group 17 (Group VIIa) of the periodic table.
| atomic number | 35 |
|---|---|
| atomic weight | [79.901, 79.907] |
| melting point | −7.2 °C (19 °F) |
| boiling point | 59 °C (138 °F) |
| specific gravity | 3.12 at 20 °C (68 °F) |
| oxidation states | −1, +1, +3, +5, +7 |
| electron configuration | (Ar)3d104s24p5 |
History
Bromine was discovered in 1826 by the French chemist Antoine-Jérôme Balard in the residues (bitterns) from the manufacture of sea salt at Montpellier. He liberated the element by passing chlorine through an aqueous solution of the residues, which contained magnesium bromide. Distillation of the material with manganese dioxide and sulfuric acid produced red vapours, which condensed to a dark liquid. The similarity of this procedure to that for making chlorine suggested to Balard that he had obtained a new element similar to chlorine. (The German chemist Justus von Liebig appears to have obtained the element before Balard, but he wrongly considered it to be iodine chloride.) Because of the bad odour of the element, the French Academy of Sciences suggested the name bromine, from the Greek word bromos, meaning “bad smell” or “stench.”
Occurrence and distribution
Number Of P Electrons In Bromine Atom
A rare element, bromine is found in nature dispersed throughout Earth’s crust only in compounds as soluble and insoluble bromides. Some enrichment occurs in ocean water (65 parts per million by weight), in the Dead Sea (approximately 5 grams per litre [0.7 ounce per gallon]), in some thermal springs, and in rare insoluble silver bromide minerals (such as bromyrite, found in Mexico and Chile). Natural salt deposits and brines are the main sources of bromine and its compounds. Jordan, Israel, China, and the United States led the world in bromine production in the early 21st century; other important bromine-producing countries during that period include Japan, Ukraine, and India.
Natural bromine is a mixture of two stable isotopes: bromine-79 (50.54 percent) and bromine-81 (49.46 percent). Of the 17 known radioactive isotopes of the element, bromine-77 has the longest half-life (57 hours).
Physical and chemical properties
Free bromine is a reddish brown liquid with an appreciable vapour pressure at room temperature. Bromine vapour is amber in colour. Bromine has a pungent odour and is irritating to the skin, eyes, and respiratory system. Exposure to concentrated bromine vapour, even for a short time, may be fatal. Like the other halogens, bromine exists as diatomic molecules in all aggregation states.
About 3.41 grams (0.12 ounce) of bromine dissolve in 100 millilitres (0.1 quart) of water at room temperature. The solution is known as bromine water. Like chlorine water, it is a good oxidizing agent, and it is more useful because it does not decompose so readily. It liberates free iodine from iodide-containing solutions and sulfur from hydrogen sulfide. Sulfurous acid is oxidized by bromine water to sulfuric acid. In sunlight bromine water decomposes, with release of oxygen, as in the following equation:
From bromine water a hydrate (a clathrate) can be isolated that contains 172 water molecules and 20 cavities capable of accommodating the bromine molecules. Bromine dissolves in aqueous alkali hydroxide solutions, giving bromides, hypobromites, or bromates, depending on the temperature. Bromine is readily extracted from water by organic solvents such as carbon tetrachloride, chloroform, or carbon disulfide, in which it is very soluble. In the organic solvents it gives an orange solution.
The electron affinity of bromine is high and is similar to that of chlorine. It is, however, a less powerful oxidizing agent, chiefly because of the weaker hydration of the bromide ion as compared with the chloride ion. Similarly, a metal-bromine bond is weaker than the corresponding metal-chlorine bond, and this difference is reflected in the chemical reactivity of bromine, which lies between that of chlorine and that of iodine. An organic bromo compound resembles the corresponding chloro derivative but is usually more dense, less volatile, less combustible, and less stable.
Bromine combines violently with the alkali metals and with phosphorus, arsenic, aluminum, and antimony but less violently with certain other metals. Bromine displaces hydrogen from saturated hydrocarbons and adds to unsaturated hydrocarbons, though not as readily as chlorine does.
The most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, Br2), +1 (hypobromite, BrO−), +3 (bromite, BrO−2), +5 (bromate, BrO−3), and +7 (perbromate, BrO−4) are also known. The first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands, mainly oxygen and fluorine. Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds.
What Is The Total Number Of Electrons In Bromine
Production and use
The chief commercial source of bromine is ocean water, from which the element is extracted by means of chemical displacement (oxidation) by chlorine in the presence of sulfuric acid through the reaction
The product of the reaction is a dilute solution of bromine, from which the element is removed by blowing air through it. The free bromine is then mixed with sulfur dioxide, and the mixed gases are passed up a tower down which water is trickling. The following reaction takes place in the tower:
resulting in a mixture of acids that is much richer in bromide ion than seawater. A second treatment with chlorine liberates bromine, which is freed from chlorine and purified by passage over moist iron filings.
Commercial bromine generally contains up to 0.3 percent chlorine. It is usually stored in glass bottles or in barrels coated with lead or Monel metal.
The industrial usage of bromine had been dominated by the compound ethylene bromide (C2H4Br2), which once was added to gasoline with tetraethyl lead to prevent deposition of lead in the engine. Since the renunciation of leaded gasoline, bromine compounds have mainly been used in flame retardants, but ethylene bromide is still an important compound because of its use to destroy nematodes and other pests in soils. Bromine is also used in the production of catalysts, such as aluminum bromide.
Bromine has other uses, as in making various dyes and the compounds tetrabromoethane (C2H2Br4) and bromoform (CHBr3), which are used as liquids in gauges because of their high specific gravity. Until the development of barbiturates in the early 20th century, bromides of potassium, sodium, calcium, strontium, lithium, and ammonium were used widely in medicine because of their sedative action. Silver bromide (AgBr), an important component of photographic film, is, like silver chloride and iodide, light sensitive. Traces of potassium bromate (KBrO3) are added to wheat flour to improve baking. Other bromine compounds of significance include hydrogen bromide (HBr), a colourless gas used as a reducing agent and a catalyst in organic reactions. A solution of the gas in water is called hydrobromic acid, a strong acid that resembles hydrochloric acid in its activity toward metals and their oxides and hydroxides.
Bromine Number Of Protons
- key people
- related topics
